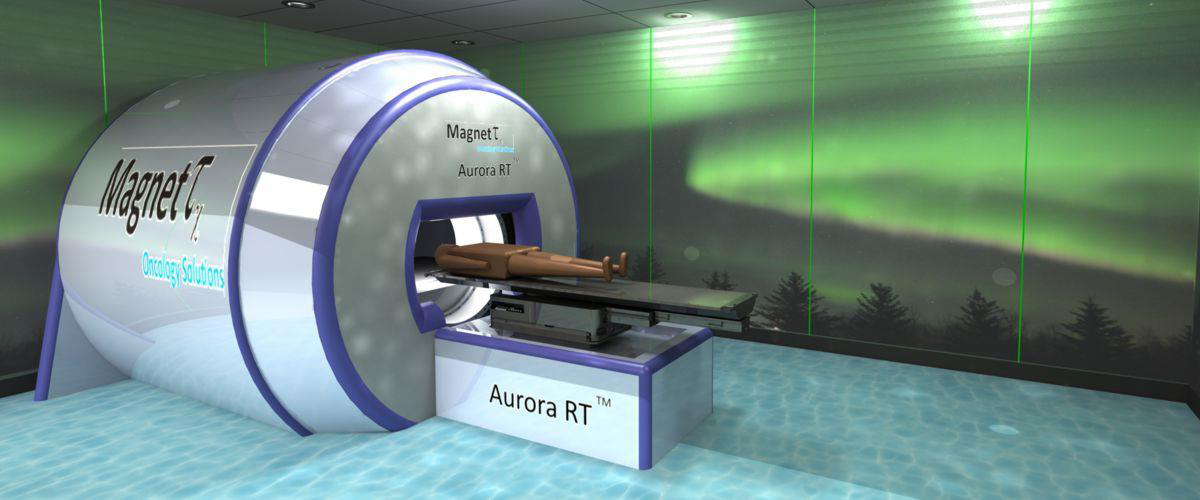
· Breakthrough cancer radiotherapeutic real-time precision device addressing a significant market need – the Aurora RT™
· Leading management and scientific team with deep relationships in the radiotherapy and medical physics space and hospital experience
· De-risked with a proof-of-concept prototype in 2008 and 2nd full working prototype in 2014 utilizing ~M of non-dilutive Canadian research grants
· Responding to RFPs for research units and addressing customer interest globally
· Raising first round of M to complete the commercial version, regulatory approval and first sale
· 2016 TEC VenturePrize DynaLIFE DX Health Award winner
|
√
|
ACCURACY
|
Optimized Magnetic Field strength & orientation
|
|
√
|
ACCURACY
|
Effectively treat peripheral tumours (e.g. breast)
|
|
√
|
ACCURACY
|
No radioactive sources used
|
|
√
|
COST/SAFETY
|
No cryogen/liquid-helium required
|
|
√
|
COST/EFFICIENCY
|
Ease of installation and maintenance
|
· Completion of commercial version (2017)
· Regulatory approvals (2017)
· Product first sale (2017)

